Injectrode Removal
To find out if neuromodulation is an option earlier in the pain treatment timeline, patients need a simple way to try it, which means simple device removal is critical.
Easy in. Easy Out.
When this Injectrode is pulled, each individual coil detaches from the surrounding tissue, retracting into the device's hollow core. This action disperses the removal force along a structured inclined plane, minimizing localized stress on any given tissue point. This innovative “unzipping” allows for full device removal from the body, and most importantly, it enables fully reversible neuromodulation.
High Tensile Strength ÷ Low Removal Force = Reliable Removal
The high tensile strength of the Injectrode (32.90 ±2.09N) compared to its low removal force (3.63 ±1.70N) creates a safety factor of 9.06. This means the force required to break the device is about 9 times greater than the force needed to remove it from tissue, ensuring safe and reliable explanation.
To us, a fully reversible treatment means nothing is left behind. No lasting side effects from drugs, no damage to your body. Just try it. Just pull it.
VISION
No more sutures, no more visible scars
We envision a world where trying neuromodulation to address chronic pain and other chronic conditions is as easy as receiving a drug injection. To realize this ambition, it's essential that scarring from stitches and sutures become a thing of the past. Both the implantation and, when deemed necessary by the patient or physician, removal of devices like the Injectrode should uphold this standard of simplicity and minimal invasiveness.
INJECTRODE TODAY
Removal via 5mm cut and steri strips
A minor incision, typically ranging from 3 to 5 mm, is made to provide forceps access to the Injectrode, enabling a simple removal.
INJECTRODE TOMORROW
Removal via needle
Work is ongoing to enable Injectrode removal through an 18 gauge needle. This is to eliminate the scalpel from the equation.
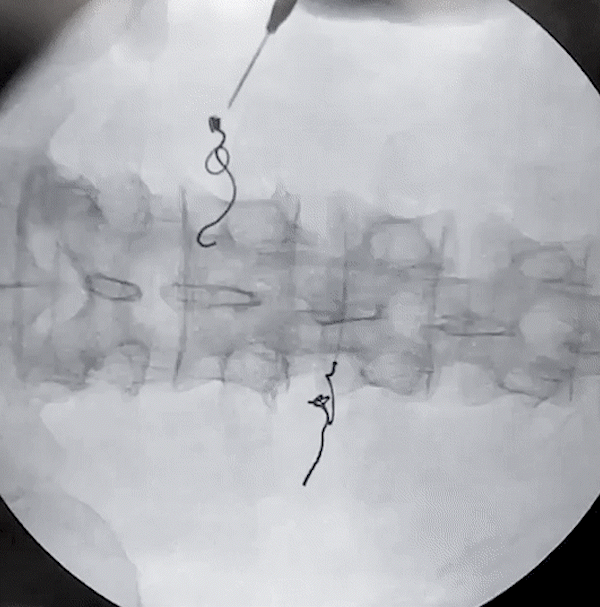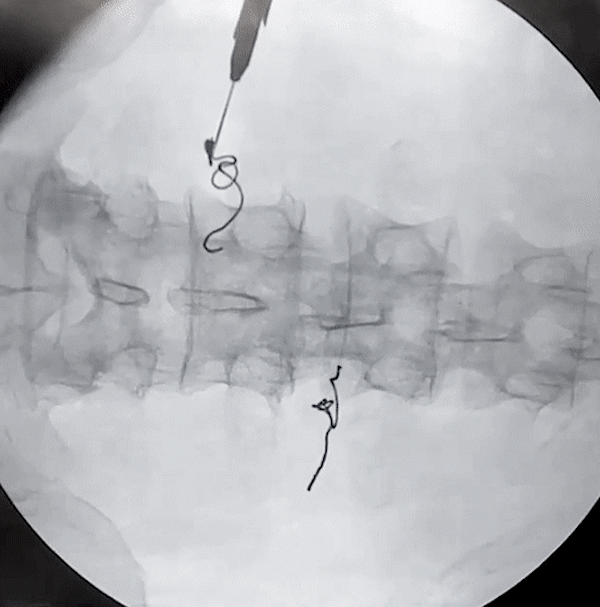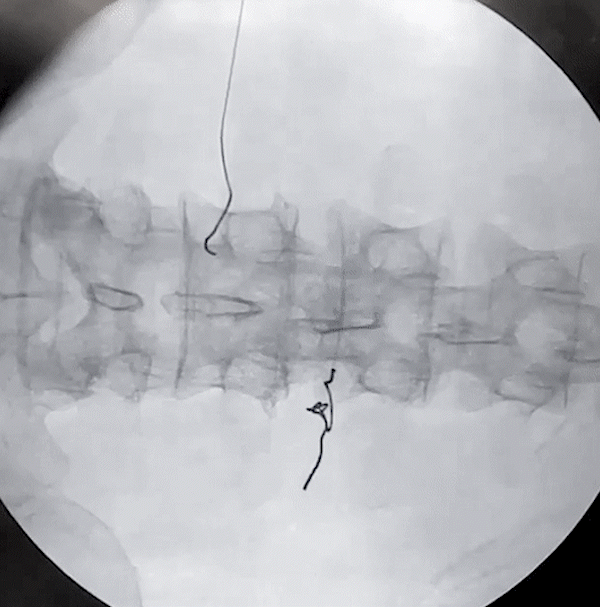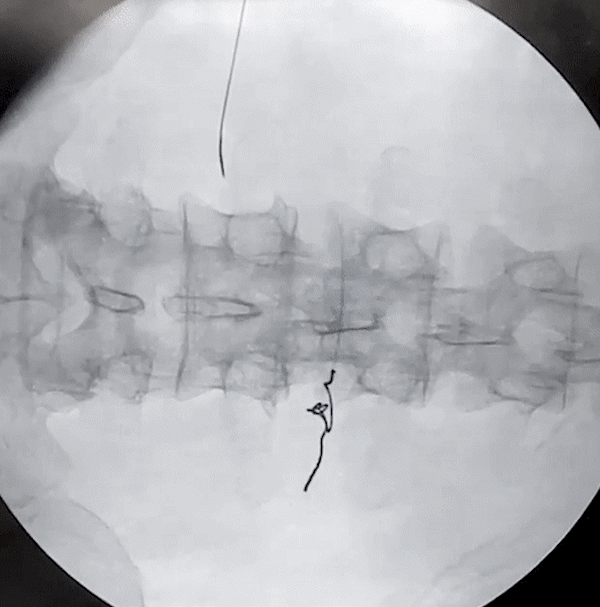
Lower Back and Posterior Tibial Nerve Removal
(via needle)
Posterior Tibial Nerve Removal
(via forceps)
porcine model
Lower Back Removal
(via forceps)
Patient safety is always at the forefront
The removal of the Injectrode prioritizes safety at its core. As the helical Injectrode elongates during extraction, each coil individually retracts into the device's inherent hollow core, minimizing its contact with surrounding tissues and ensuring a safer removal. This action evenly disperses the removal force along an inclined plane, reducing the chances of exerting undue stress on a specific tissue area.
Such an approach not only safeguards the tissues but also greatly reduces the risk of potential device malfunctions or fractures during extraction. The hollow core design plays a pivotal role in distributing these forces, reinforcing both the safety and effectiveness of the Injectrode's removal process.
Note how the Injectrode leaves behind an imprint of its helical structure, as seen in a hydrogel model. This is evident in both its linear and the bundled anchor configurations. The Injectrode unzips one loop after another to achieve this effect, allowing for the removal of the anchor loop by loop.
The Injectrode was designed with removal in mind
This is a feature that many other implanted devices are not necessarily optimized for, requiring much larger surgical access wounds that rely on sutures to be closed.